An effective anti-emetic active pharmaceutical ingredient, maropitant citrate helps to ensure that our four-legged friends continue to be healthy and content. It is also known as maropitant. This comprehensive guide, which was produced by the well-known supplier Qingmu Pharmaceutical, will cover all of the information that there is to know about Maropitant citrate. It will cover absolutely everything there is to know about it.
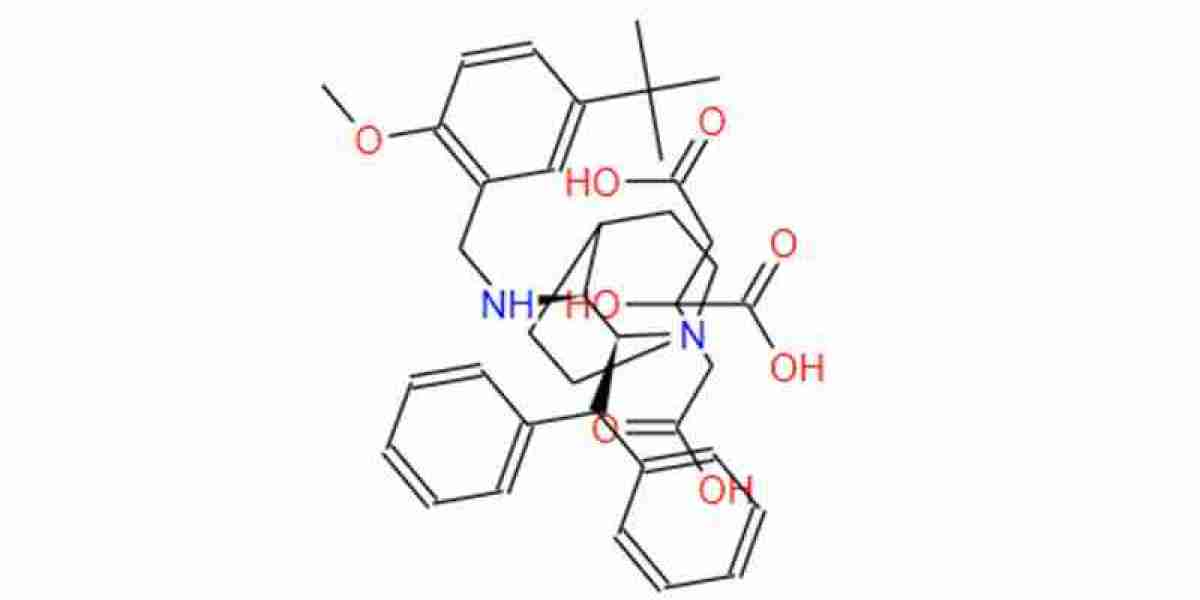
To what does the term "Maropitant Citrate" refer?
A prescription is required to obtain Cerenia, which is a medication that is extremely effective and can be administered to both cats and dogs. In a wide variety of conditions, maropitant citrate has the potential to be an effective treatment and preventative measure for instances of acute vomiting. Maropitant citrate is a medication that helps improve the quality of life for millions of pets whose illnesses are related to vomiting each year. This medication is used to treat vomiting-related illnesses. There are 374 grams in one mole. Furthermore, there are medication dosage forms that can be administered via injection for the treatment of acute vomiting. Authorized by the Food and Drug Administration (FDA) for use in the United States and widely distributed across the globeAdditionally utilized for the purpose of alleviating nausea following surgical procedures, the adverse effects of chemotherapy, and other conditions of a similar nature.
In doses ranging from 0 to 10. Taking into consideration the fact that Qingmu Pharmaceutical is a leading maropitant manufacturer, the company offers the following advantages for development projects and bulk purchasing:
Manufacturing facilities that have been approved by the Food and Drug Administration (FDA), the International Organization for Standardization (ISO), the European Medicines Agency (EMA), and other major regulatory bodiesFor more than ten years, we have been specializing in the production of veterinary active pharmaceutical ingredients (APIs). Multiple tons of maropitant citrate can be produced annually; this is the capacity of the production facility. Achieving compliance with the pharmacopeia standards of the European Union, Japan, and the United States or CanadaPrices that are 15–30% lower than the average in the market and are competitive with other businessesExpress shipping is available for orders that are considered to be urgent as well as for local warehouses. The results can be verified through the use of free samples that are available for testing. Due to the fact that they have an impeccable compliance record, they guarantee a continuous supply of maropitant citrate API that is of the highest possible purity.
With the help of highly selective catalysts, it is possible to produce the desired enantiomer with an efficiency that is greater than 99% and without producing any waste. For the purpose of preventing decomposition, the drying and milling process employs a vacuum or fluid bed drying method while maintaining a controlled temperature. The filling process makes use of automated weighing and capping in a cleanroom that is well separated in order to achieve the goal of achieving a high level of output. For the purpose of ensuring that specifications such as ICH specifications are adhered to and that consistency and purity standards are met, each batch is put through over forty parametric chemical and physical assays, in addition to specifications testing. This is done in order to guarantee that the specifications are met. It is necessary to comply with Good Manufacturing Practice (GMP) and transport safety regulations by ensuring that containers and drums are appropriately labeled with specific information. The maropitant citrate that is produced by this meticulous and straightforward synthesis, which has been validated for use on a large scale, has a purity of 99%, and the residual solvents are significantly lower than the permittivity level.
The implementation of quality control measures by Qingmu Pharmaceuticals ensures that a supplier's reputation for continuously delivering dependable supply is preserved. Environmental management in accordance with the guidelines of ISO 14001 for API Good Manufacturing Practice in accordance with ICH Q7Provision of certifications for food facilities in accordance with Kosher, Halal, and FSSAI standardsapprovals from the most significant regulatory authorities on a global scale that CPI, PDA, and other industry organizations are among the organizations that require membership that are among the organizations that require membership. The manufacturing facility that they have is situated in Chengdu, China, and it is outfitted with fully automated production lines that have been specifically designed for the production of APIs that are strictly controlled under controlled conditions from A to Z.
Costs and the Presentation of the ProductThe price of Qingmus maropitant citrate API is typically between 15 and 30 percent lower than the prices of competitors in the market. This is because economies of scale allow for the production of a larger quantity of the product. The pure white powder is packaged in a manner that ensures its hygienic quality by utilizing a multilayered container that consists of an inner polyethylene bag with a sealed cap, an inner fiber drum with air cushion, and an outer carton for strengthened containment. This container is used to package the powder. Because of the expertise of logistical professionals who make use of GSP, DDP, and other INCOTERMS, it is possible to guarantee that deliveries will be made on time anywhere in the world.